Description
During heating samples often undergo phase transitions and/or weight change due to evaporation of solvents and/or chemical reactions. These changes can be detected by thermal analysis: calorimetric techniques (DTA and DSC) give information about the heat involved in these processes and thermogravimetry (TG) shows the weight change.
Weight change can be either weight increase due to oxidation reactions or weight loss due to decomposition by liberation of volatile compounds. Analysis of these evolved gases can give valuable information about the sample composition and reaction pathways for decomposition. As thermal analysis gives no information about the nature of the evolved gases, coupling with spectrometers or chromatographs is a valuable tool for evolved gas analysis (EGA).
Applications
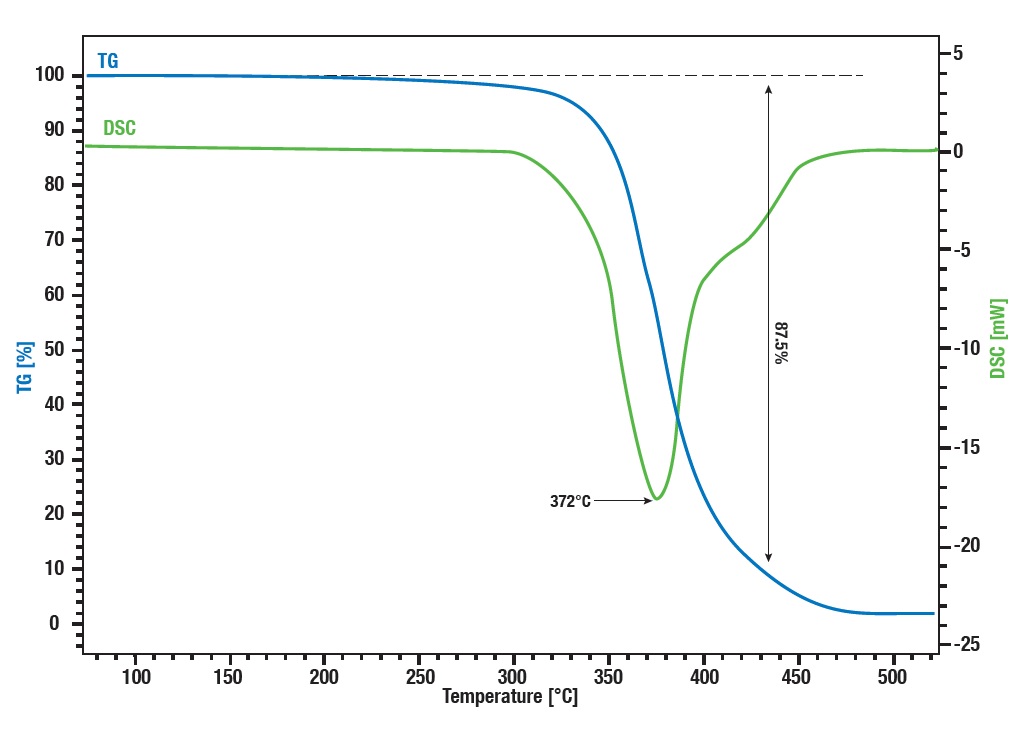
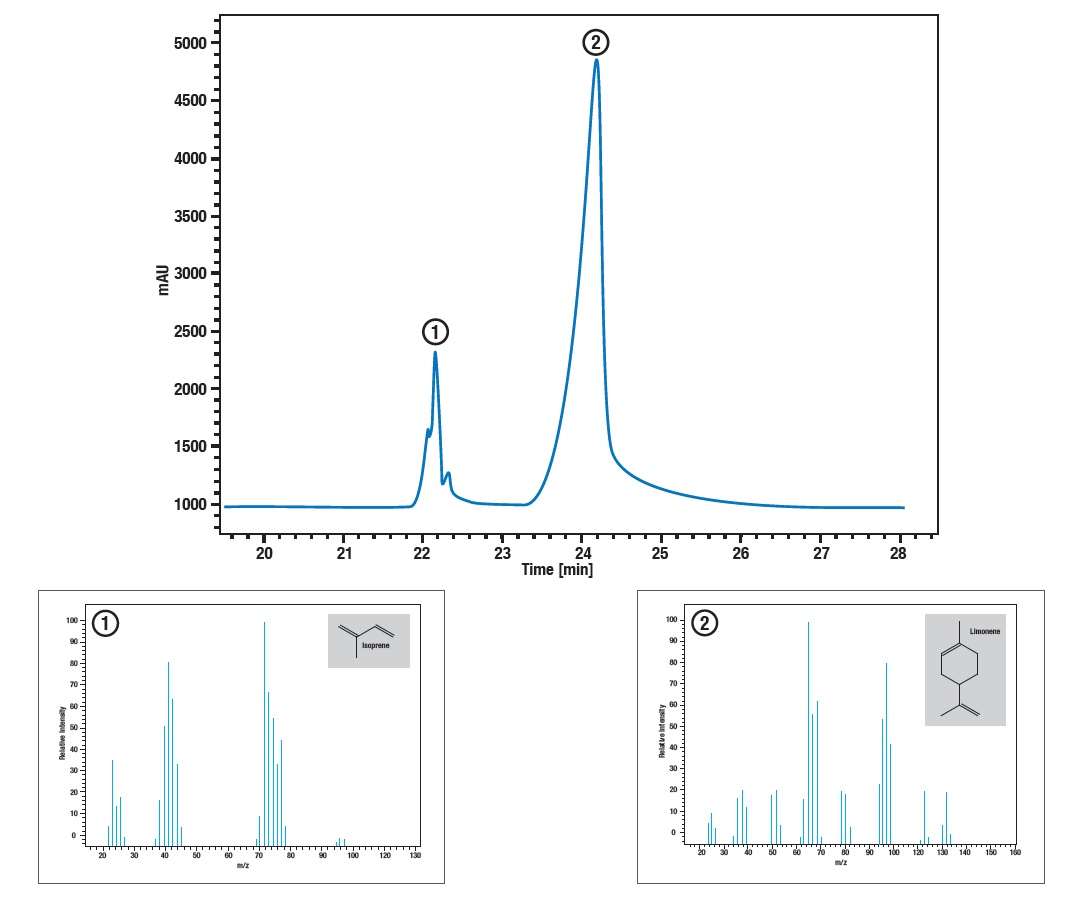
Application example: Thermal decomposition of latex
At 370° synthetic rubber decomposes into some monomer parts. The main parts limonene and isoprene can be identified using STA combined with GC-MS. The STA signal shows mass loss and enthalpie change at 372°. At the same time the GC shows two peaks, a smaller and a bigger one, where the samller one can be identified by mass spectrometrie as isoprene and the bigger one as limonene.

 SingaporeSG
SingaporeSG ChinaCN
ChinaCN MalaysiaMY
MalaysiaMY IndonesiaID
IndonesiaID MyanmarMM
MyanmarMM



