Overview
Lullaby is the ideal siRNA transfection reagent for gene silencing. Relying on the TEE-technology, it has been successfully tested on numerous cell lines, reaching up to 90% gene silencing with high reproducibility and a very low toxicity. RNA interference is a powerful technique to shut down genes expression in cells and organisms. This silencing effect constitutes a very helpful tool to study gene’s function and a promising approach for new therapeutic treatments. Short RNA duplexes (siRNA: small interfering RNA, shRNA: small hairpin RNA and dsRNA: double strand RNA) are extremely selective by interacting and inducing the degradation of their specific mRNA targets and thereby inhibit the resulting protein production. Lullaby® siRNA Transfection Reagent introduces the siRNA duplexes in a variety of cells with a very high efficiency leading to exceptional knockdown effects with low doses of siRNA.
"We initially collated a transfection reagent library of 26 reagents...By far, our preferred reagent is Lullaby from OZ Biosciences. We have used this reagent in over 20 cell lines and have found it essential in enabling siRNA screens in hard to tranfect cell lines..., with minimal toxicity"
Emma L. Shanks (2014) Strategic siRNA Screening Approaches to Target Cancer at the Cancer Research UK Beaston Institute, Combinatorial Chemistry & High Throughput Screening, 17:328-332
- Effective with very low doses of siRNA
- Minimized off-target effects
- Suitable for siRNA, shRNA, dsRNA, etc.
- Applicable to a broad range of cells
- Serum compatible & Non toxic
- Straightforward protocol
Sizes:
- 500 µL: Up to 1000 assays
- 1000 µL: Up to 2000 assays
- 3 x 1000 µL: Up to 6000 assays
Storage: + 4°C
Shipping conditions: Room temperature
| CATALOG NUMBER |
UNIT SIZE |
| LL70500 |
500 µL
|
| LL71000 |
1 mL
|
| LL73000 |
3 mL
|
Applications
- Perfect for all gene silencing apllications: siRNA, shRNA, miRNA, dsRNA
- Suitable for all mammalian cells: Cell lines, hard-to-transfect & primary cells
RECOMMENDED FOR: siRNA transfection of cell lines. Perfect for High-Throughput Screening
Results
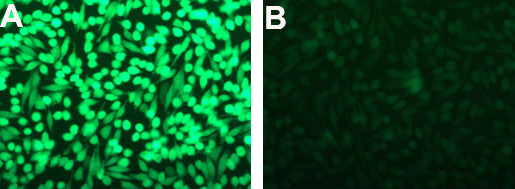
Figure 1. GFP silencing in HeLa cells. GFP-expressing HeLa cells (A) seeded in a 24-well plate were transfected with 1μL Lullaby + 5nM (33.75ng) siRNA (B) . GFP extinction was monitored 72h post-transfection by fluorescence microscopy.
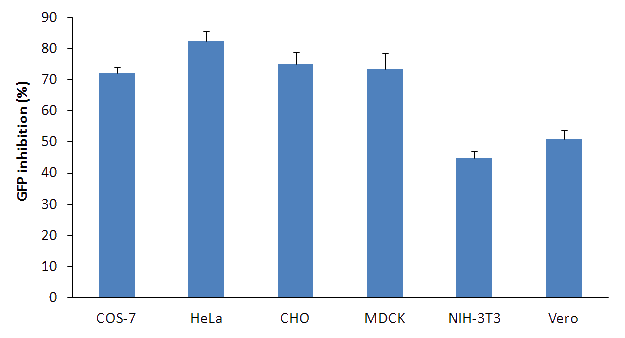
Figure 2. GFP silencing in various cell lines with Lullaby GFP-expressing cells were seeded on a 24-well plate and transfected with 10nM (67.5ng) siRNA associated with 2μL of Lullaby. GFP extinction was monitored 72h post-transfection by flow cytometry.

 SingaporeSG
SingaporeSG ChinaCN
ChinaCN MalaysiaMY
MalaysiaMY IndonesiaID
IndonesiaID MyanmarMM
MyanmarMM_pddqnqv8.png)




_9vekyf0h.jpg)
_zkyhdq4e.jpg)
_pogkev30.jpg)